Article published on PR NEWSWIRE
Click here to read the full article at prnewswire.com
CAROLINA, Puerto Rico, May 14, 2024 /PRNewswire/ — CorePlus www.corepluspr.com, a precision pathology organization, announced today that it is the first laboratory in the world to operationalize the Techcyte AI algorithm and quality control workflow for cytology-based cervical cancer screening. After more than two years of clinical collaboration with Techcyte, we have achieved the level of precision and accuracy necessary to deploy this tool for use with our patients to provide 100% quality control of all Pap tests, which goes well beyond the regulatory mandates.

We are fortunate to have been able to include Puerto Rican women in the validation of the algorithm. Ethnic diversity is a key to eliminating potential algorithmic bias. Including this subset of the population, ensures that Hispanic women will be well represented. This is important as Hispanics are becoming a significant percentage of the US population.” said Mariano de Socarraz, Founder and CEO of CorePlus.
Puerto Rican women faced an increase in the incidence of cervical cancer rate from 9.2 to 13 cases per 100,000 between 2001 to 20171. Early detection is paramount to provide initial therapeutic intervention and ensure better quality of life and impact healthcare cost.
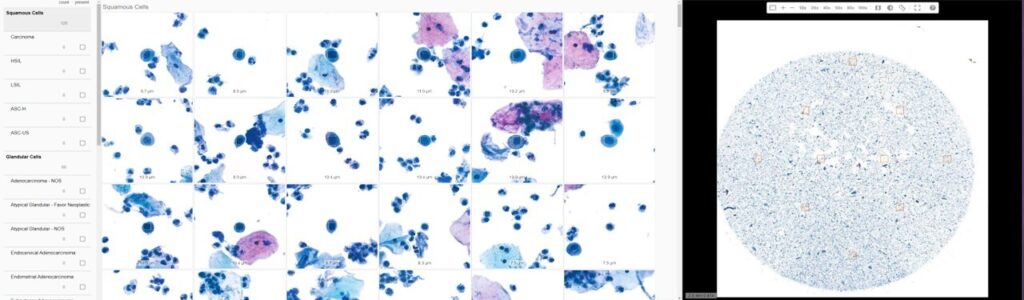
Juan C. Santa-Rosario, M.D., CorePlus’ Chief Medical Officer states, “Our validation study included digitization of more than 1,500 cases scanned on the Epredia P1000 with water immersion at different modalities and was conducted by Dr. María del Mar Rivera-Rolón, our Board-Certified Cytopathologist. Now we will be able to review 100% of Pap tests processed at our laboratory using the Techcyte AI-assisted tool with the intent of providing more accurate results and promoting patient safety.”
“CorePlus is one of the most innovative organizations in the world at implementing digital pathology solutions. We are thrilled they will be using our AI-based cervical cytology software to have a real impact on the health of women in Puerto Rico and throughout the world. We appreciate their collaboration and shared mission to make a positive impact for lab professionals and the patients they serve.” said Ben Cahoon, CEO of Techcyte.
CorePlus is committed to deploying new and disruptive technologies that will benefit our patients, physicians, and the healthcare ecosystem at large.
About CorePlus
CorePlus Servicios Clínicos y Patológicos, LLC, a precision pathology organization, a leader in the transformation of pathology to digital pathology and the use of Artificial Intelligence (AI) algorithms since 2020. CorePlus is a high complexity CLIA certified laboratory with facilities in Carolina and Ponce, Puerto Rico. CorePlus has implemented and validated the AI-powered solutions pursuant to CLIA laboratory developed test (LDT) regulations and College of American Pathology (CAP) guidelines. The CorePlus Team of Board-certified pathologists became the first in the United States and the Americas to operationalize Ibex’s Galen Prostate solution June of 2020.
Visit www.corepluspr.com for more information.
About Techcyte
Founded in 2013, Techcyte is transforming the practice of pathology with a unified pathology platform that digitizes lab workflows and offers AI tools that improve the efficiency and accuracy of diagnostic testing.
Our mission is to positively impact the health of humans, animals, and the environment through the use of artificial intelligence.
We do that by partnering with best-in-class labs, whole slide scanner manufacturers, AI vendors, diagnostic companies, hardware manufacturers, and solution providers. Together, we aim to deliver a unified clinical and anatomic pathology platform to labs and clinics around the world.
Visit www.techcyte.com for more information.
About Epredia
Epredia, a global leader in precision cancer diagnostics and subsidiary of PHC Holdings Corporation (TSE: 6523) offers a unique array of end-to-end instruments for pathology workflows including tissue processors, microtomes, embedding stations, and a range of 3DHistech developed whole slide scanners from the single slide Pannoramic Desk II to the Pannoramic 1000 scanner. These scanners offer a single slide to 1000 slide capacity and a throughput of up to 85 slides per hour, including overnight scanning.
Visit www.epredia.com for more information.
Ortiz, A.P., Ortiz-Ortiz, K.J., Colon-Lopez, V., Tortolero-Luna, G., Torres-Cintron, C.R.,Wu, C.F., Deshmukh, A.A., 2021. Incidence of Cervical Cancer in Puerto Rico, 2001-2017. In JAMA Oncology (Vol. 7, Issue 3, pp. 456–458). American Medical Association. 10.1001/jamaoncol.2020.7488.
SOURCE CorePlus Servicios Clinicos y Patologicos, LLC